

8 titration flasks titrating apparatus Timer
Pipette with its safety-filler
A=60 cm30.02M I2 B=30cm3 1M propanone
C=30cm31M sulphuric acid D=100ml 0.5M NaHCO3
E=200ml thiosulphate(0.01M)
1. Set up titration apparatus and fulfil with 0.010M sodium thiosulphate.
2. Label titration flasks 1,2,3,45,6,7,8.
3. In 1 put 50 cm3
of 0.02M I2 in KI.
4. In 2 put 25 cm3 of aqueous propanone (CH3COCH3) and 25 cm3 of 1M H2SO4.
5. Into remaining 6 put 10cm3 of 0.5M NaHCO3(sodium hydrogencarbonate).
6. Start time, pour flask 2 into flask 1 and shake for 1 minute.
7. Withdraw using pipette + safety filler & run into flask 3.
8. Shake until bubbling ceases, note time (record below).
9. Put a bit 1% starch into the liquid (starch makes I2 black and thus titration easier).
10. Titrate with 0.010M sodium thiosulphate (Na S2O3).
11. Repeat last three at
every 5-10 min.
|
Flask No. |
Time |
Initial burette reading |
Final burette reading |
Amount of sodium hydrogencarbonate used |
|
3 |
1.10 |
0 |
19.5 |
19.5 |
|
4 |
6.20 |
20 |
39.2 |
19.2 |
|
5 |
11.33 |
0 |
17.5 |
17.5 |
|
6 |
14.50 |
17.5 |
35.3 |
17.8 |
|
7 |
20.07 |
0 |
14.5 |
14.5 |
|
8 |
24.15 |
14.5 |
30.1 |
15.6 |
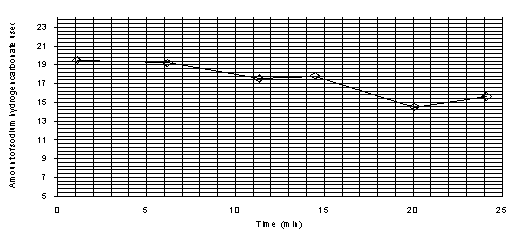
The line of best fit will be a straight line, thus reaction is zero order wrt. I2.
4 boilingtubes in holder burette with holder Timer
100 cm3 2M HCl 50cm32M propanone 20cm3 H2O
50cm30.01M I2 20cm3 and 10cm3 pipettes with filler beakers
1. Set up testtubes and label beakers and fill them.
2. Make up mixtures below
3. Start reaction by adding I2.
4. Measure time for colour of I2 to disappear.
|
Volume |
Run1 |
Run2 |
Run3 |
Run4 |
|
2M HCl (cm3) |
20 |
10 |
20 |
20 |
|
2M propanone (cm3) |
8 |
8 |
4 |
8 |
|
water (cm3) |
0 |
10 |
4 |
2 |
|
0.01M I2 (cm3) |
4 |
4 |
4 |
2 |
|
Time for colour to disappear(s) |
78 |
205 |
182 |
55 |
|
Rate (1000x1/T) |
12.8 |
4.88 |
5.49 |
18.2 |
Rate=kx
Water was added to keep the total volume constant, thus making it a fair test.
1. Compare run 1&2, if the concentration of acid is halved the rate is also halved.
2. Compare run 1&4 for how the I2 changes the rate does not change (note: the rate actually increases, but that is because the iodine colour gets harder to notice, because there is less iodine).
3. Compare runs 1&3 for how the propanone changes the rate of reaction-rate is halved if concentration is halved.
Overall
r=k[H+]1[CH3COCH3]1[I2]0.
Overall order: Second.