25.0cm3 of the 1M various acids. 100cm3 beakers
1.0 molar alkalis (75cm3) timer
magnetic stirrer Titrating apparatus with burette
Pipette and filler pH meter
Put 25cm3 of acid into beaker by pipette
Stand the beaker to stirrer
Connect the pH meter
Place the burette above beaker and fill it with alkali
Start stirrer and start titrating noting the time and pH (use35 cm3 of alkali altogether)
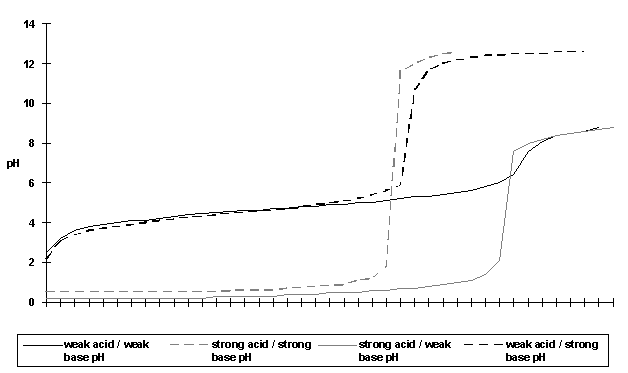
|
cm3 of alkali added |
weak acid / weak base pH |
strong acid / strong base pH |
strong acid / weak base pH |
weak acid / strong base pH |
|
0 |
2.5 |
0.5 |
0.2 |
2.2 |
|
1 |
3.2 |
0.5 |
3.1 |
|
|
2 |
3.6 |
0.5 |
3.4 |
|
|
3 |
3.8 |
0.5 |
3.6 |
|
|
4 |
3.9 |
0.5 |
||
|
5 |
4.0 |
0.5 |
||
|
6 |
4.1 |
0.5 |
||
|
7 |
4.1 |
0.5 |
||
|
8 |
4.2 |
0.5 |
||
|
9 |
4.3 |
0.5 |
||
|
10 |
4.4 |
0.5 |
4.3 |
|
|
11 |
4.45 |
0.5 |
0.2 |
4.3 |
|
12 |
4.5 |
0.5 |
||
|
13 |
4.55 |
0.6 |
4.5 |
|
|
14 |
4.6 |
0.6 |
4.5 |
|
|
15 |
0.6 |
|||
|
16 |
4.7 |
0.6 |
0.3 |
|
|
17 |
0.7 |
|||
|
18 |
4.8 |
0.7 |
||
|
19 |
0.8 |
0.4 |
||
|
20 |
4.9 |
0.8 |
||
|
21 |
0.9 |
|||
|
22 |
5.0 |
1.1 |
0.5 |
5.2 |
|
23 |
1.2 |
|||
|
24 |
5.1 |
1.8 |
0.6 |
5.6 |
|
25 |
5.2 |
11.6 |
5.9 |
|
|
26 |
5.3 |
12.0 |
10.7 |
|
|
27 |
5.3 |
12.3 |
11.7 |
|
|
28 |
5.4 |
12.5 |
12.0 |
|
|
29 |
5.5 |
12.6 |
12.2 |
|
|
30 |
5.6 |
1.1 |
12.3 |
|
|
31 |
5.8 |
1.4 |
12.4 |
|
|
32 |
6.0 |
2.1 |
||
|
33 |
6.4 |
7.6 |
||
|
34 |
7.6 |
8.0 |
||
|
35 |
8.1 |
8.2 |
||
|
36 |
8.4 |
8.4 |
||
|
37 |
8.5 |
12.6 |
||
|
38 |
8.6 |
|||
|
39 |
8.8 |
|||
|
40 |
8.8 |